Please ENTER to search
Please ENTER to search
5908-99-6
Muscarinic antagonist
US DMF Assessment Completed & CEP Approved regulatory_status
CEP [R1 CEP 2015-317- Rev 01] EDMF Available
ISOPTO ATROPINE
Ophthalmology, Muscarinic antagonist
#029605
TGA Approved, EDMF Approved in Portugal Agency, Infarmed, DMF Approved in CFDA, China
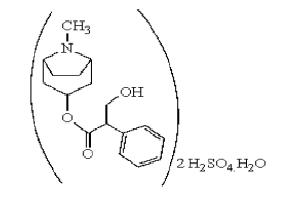
Atropine Sulfate is an antimuscarinic agent that works by blocking the muscarinic acetylcholine receptors in the smooth muscles, secretory glands, and the central nervous system. It competitively inhibits the action of acetylcholine, a neurotransmitter that activates the parasympathetic nervous system. This results in decreased parasympathetic activity, leading to effects such as increased heart rate, decreased salivation, dilated pupils, and bronchial muscle relaxation. Atropine Sulfate also crosses the blood-brain barrier, exerting central nervous system effects.
Atropine Sulfate is primarily used in the treatment of bradycardia (low heart rate), certain types of heart block, and to reduce salivation and bronchial secretions during surgery. It can also be used as an antidote for organophosphate poisoning, in eye examinations to dilate pupils, and for symptomatic relief of gastrointestinal disorders like hypermotility and spasticity.
Disclaimer: Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement. The liability of any sale into such protected markets is at buyer's risk.

Request you to provide all details mentioned in the form along with your business requirement. It will help us route your query to the appropriate person.